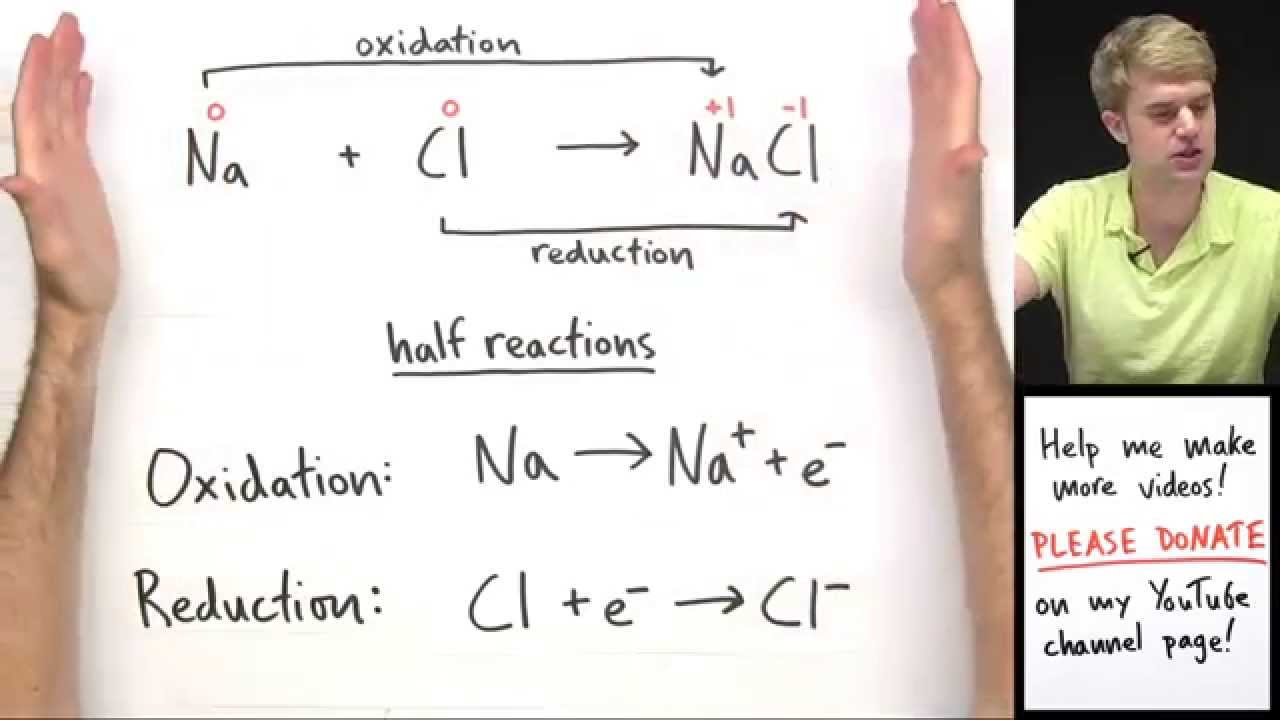
 This is an introduction to oxidation reduction reactions, which are often called redox reactions for short. An oxidation reduction (redox) reaction happens when electrons are transferred between atoms. A loss of electrons is called oxidation, and we say that atom has become oxidized. A gain of electrons is called reduction, and we say that the atoms has become reduced. The two separate parts (oxidation and reduction) of an oxidation reduction (redox) reaction are called half reactions. Two half reactions can be put together to make the whole reaction. Oxidation numbers are numbers that can be written above atoms to show whether they are gaining or losing electrons.
This is an introduction to oxidation reduction reactions, which are often called redox reactions for short. An oxidation reduction (redox) reaction happens when electrons are transferred between atoms. A loss of electrons is called oxidation, and we say that atom has become oxidized. A gain of electrons is called reduction, and we say that the atoms has become reduced. The two separate parts (oxidation and reduction) of an oxidation reduction (redox) reaction are called half reactions. Two half reactions can be put together to make the whole reaction. Oxidation numbers are numbers that can be written above atoms to show whether they are gaining or losing electrons.
Advertisement
Introduction to Oxidation Reduction (Redox) Reactions
Myahi
February 18, 2021
 This is an introduction to oxidation reduction reactions, which are often called redox reactions for short. An oxidation reduction (redox) reaction happens when electrons are transferred between atoms. A loss of electrons is called oxidation, and we say that atom has become oxidized. A gain of electrons is called reduction, and we say that the atoms has become reduced. The two separate parts (oxidation and reduction) of an oxidation reduction (redox) reaction are called half reactions. Two half reactions can be put together to make the whole reaction. Oxidation numbers are numbers that can be written above atoms to show whether they are gaining or losing electrons.
This is an introduction to oxidation reduction reactions, which are often called redox reactions for short. An oxidation reduction (redox) reaction happens when electrons are transferred between atoms. A loss of electrons is called oxidation, and we say that atom has become oxidized. A gain of electrons is called reduction, and we say that the atoms has become reduced. The two separate parts (oxidation and reduction) of an oxidation reduction (redox) reaction are called half reactions. Two half reactions can be put together to make the whole reaction. Oxidation numbers are numbers that can be written above atoms to show whether they are gaining or losing electrons.
Popular Videos
Collateral
February 24, 2021
Ariana grande - side to side ||lyrics
March 09, 2021
Nick Drake - Which Will (lyrics video)
February 20, 2021
What we EAT in a DAY as Victoria's Secret Models
February 24, 2021
Recent
6/recent/post-list
Categories
Contact Form
HOT
6/random/post-list

0 Comments